Quality Control
Quality control (QC) for haemoglobin analysers involves a set of procedures designed to ensure the accuracy, precision, and reliability of patient test results. This is achieved through both internal quality control (IQC) within the laboratory and participation in external quality assessment schemes (EQAS).
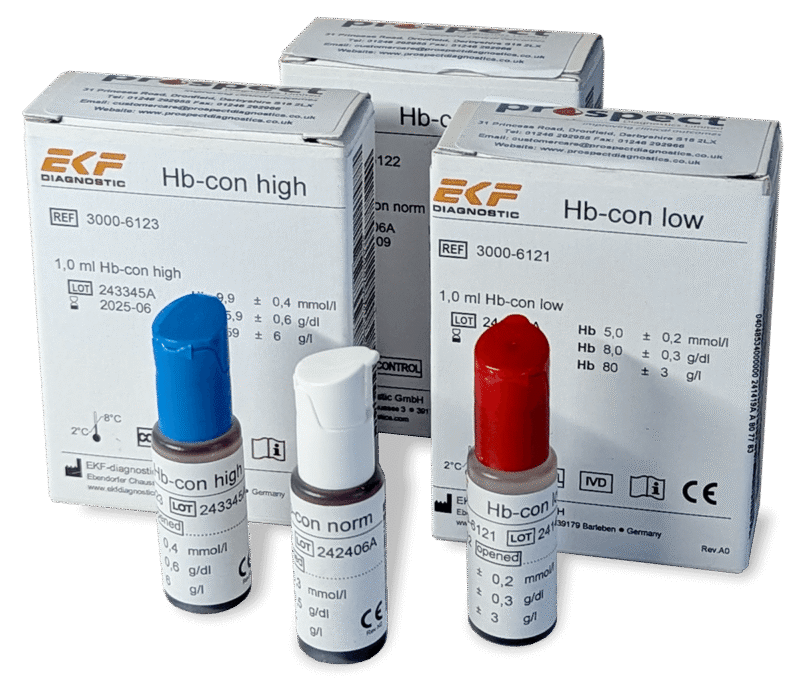
IQC involves the daily, continuous self-evaluation of the analytical process using specially prepared control materials (stabilised blood products) with known values.
Running Control Materials: Laboratories run at least two, but ideally three, levels of control materials (low, normal, and high haemoglobin concentrations) regularly (e.g., once or twice per shift).
Monitoring Results: Each time a control is run, the result is plotted on a Levey-Jennings chart. This chart helps visualise performance patterns over time, detect shifts or trends, and determine if results are within acceptable limits (usually the mean ± 2 standard deviations).
Corrective Action: If a control result falls outside the established range or exhibits a problematic pattern (e.g., consistent values on one side of the mean, or a progressive drift), patient testing is halted, the cause of the error is investigated (e.g., reagent issue, maintenance need, calibration problem), and corrective action is taken before retesting controls and patient samples.
Calibration: While not a daily QC activity, the analyser must be calibrated (or its calibration verified) upon installation, after major maintenance, or if QC results indicate a problem that cannot be otherwise resolved.
EQAS, also known as proficiency testing, involves an independent external agency sending unknown samples to different laboratories for analysis.
Peer Comparison: The laboratory’s results are compared to the results from a “peer group” of other laboratories using the same method and instrument, or to a national/international consensus value.
Benchmarking and Improvement: This process helps identify inter-laboratory differences, assess the accuracy (bias) of the lab’s method relative to others, and highlights areas for potential improvement or additional training.
Prospect Diagnostics offers you the most reliable, simplest and highly cost-effective means of monitoring the precision and accuracy of your Haemoglobin Analysers – by adopting the Eurotrol External Quality Assessment Scheme (EQAS).
Our EQA scheme allow you to post your readings to a secure online portal, where you can review your data and compare your results with Europe-wide collated data.
To find out more, please choose from the options below:
CueSee® Haemoglobin-PT External Quality Assessment Scheme
The Euro-Trol CueSee® Haemoglobin-PT External Quality Assessment Scheme (EQAS) offers you the solution to guaranteed quality. The scheme provides a monthly analysis of samples distributed simultaneously to all users facilitating performance comparison within specific ‘user groups’ and satisfying the requirements for clinical audit.